|
EXPERIMENTAL BLOOMERY IRONMAKING
in the Weald of Southern England in the 21st Century
COMPARISON with ANCIENT SLAGS
We are not just interested in producing iron blooms, we are also attempting to produce slags which look similar to those we find in the Weald. These vary in composition, but generally, if of Roman origin, they contain a high proportion of iron oxide in the form of wustite (FeO). Under the microscope, the wustite appears near white in colour, and is shaped rather like a tree, (a dendrite) or appears as a row of 'blobs' when just the 'branches' of the dendrite cut the surface being examined. The micrographs illustrate some slags found in the field and WIRG experimental slags for comparison.
Image 14: Microstructures of field find slags and WIRG experimental slags
(white phase - wustite; light grey - fayalite; black - glassy anorthite) |
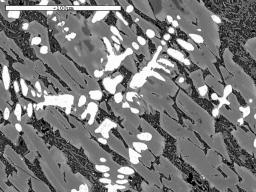
(a) Medieval? - Tudeley |
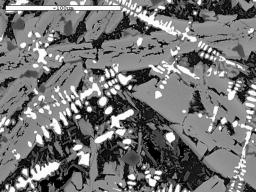
(b) Roman - Oldlands |
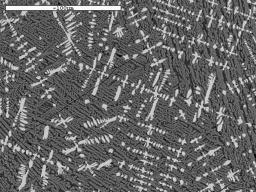
(c) WIRG smelt 20 tap slag |
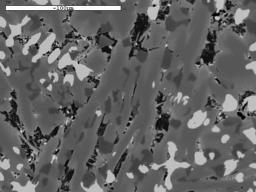
(d) WIRG smelt 20 furnace slag |
The microstructure and composition of the tap slag from our smelt 20 most closely resembles that of some of the ancient slag samples examined (Table 3). In particular, Tap Slag 20 contains a higher proportion of wustite than we have found in other experimental slags, and its chemical composition is very close to that of the Roman samples from Holtye and Oldlands. The microstructure is, however, more similar to the slag believed to be of medieval origin. To achieve this degree of similarity, the ore charge was enriched with 10% hammer scale from half way through the smelt onwards. 'Furnace Slag', which remained in the furnace and did not flow out on tapping, showed a lower proportion of iron (Fe) and silica (SiO2) present and a higher quantity of lime (CaO).
The other constituents seen in the slag microstructures are light grey needles of the iron rich olivine, Fayalite (2FeO.SiO2) all held in a glassy matrix which has a general composition (CaO.Al2O3.2SiO2), corresponding to the mineral Anorthite. The relative proportions of the constituent compounds (FeO, SiO2, CaO & Al2O3) vary as they exist as solid solutions over a range of compositions (a 'phase field') and also other elements can substitute for the main constituent elements, for example, Ca may replace some of the iron atoms in Fayalite. Other minor phases may also be present in the slag particularly if it has reached a high temperature. Examples are Pyroxenes (sometimes seen as fine needles in which Mg or Fe has replaced Ca in a silicate compound), the olivine kirschsteinite (CaFeSiO4), Hercynite (an intermediate phase between wustite and anorthite), and the potassium-containing compound, leucite K(Al2Si2O6) - the source of potassium here being the charcoal ash.
Table 3 Comparison of compositions of slag finds within the Weald with WIRG experimentally produced slags (average of five points):
| |
(Medieval ?) |
Roman
Holtye |
Roman
Oldlands |
WIRG
Smelt 20*
Tap slag |
WIRG
Smelt 20*
Furnace slag |
Compound
| %
(semiquant) |
%
(semiquant) |
%
(semiquant) |
%
(semiquant) |
%
(semiquant) |
| FeO |
37.8 |
47.0 |
47.8 |
47.8 |
43.5 |
| SiO2 |
29.4 |
28.9 |
27.3 |
27.1 |
24.5 |
| Al2O3 |
7.4 |
9.0 |
8.5 |
6.6 |
7.2 |
| CaO |
14.6 |
7.2 |
8.5 |
9.6 |
13.8 |
| K2O |
1.5 |
1.5 |
1.4 |
1.2 |
1.4 |
| Na2O |
0.9 |
0.9 |
1.0 |
1.1 |
1.1 |
| MnO |
2.6 |
2.2 |
2.2 |
2.1 |
2.5 |
| TiO2 |
0.6 |
0.5 |
0.4 |
0.3 |
0.3 |
| MgO |
3.6 |
1.3 |
1.8 |
2.8 |
3.2 |
| P2O5 |
1.1 |
0.9 |
0.7 |
0.8 |
1.1 |
| Cr2O3 |
Not detected |
Not detected |
0.1 |
Not detected |
Not detected |
*Smelt 20 Charcoal:Ore 1:1 with 10% hammer scale added from charge 7 to 20 (end)
Air rate 4-5l/sec |
Higher temperatures may have been achieved in later medieval times as bloomery furnaces became bigger and water was used to power the bellows instead of people. The slag analysis identified 'Medieval?' is believed to be of 14th-century origin.
It is interesting to note that the medieval slag in Table 3 has less iron in it and more calcium oxide (lime). The presence of lime improves the yield of the smelt, the Ca displacing Fe from the slag. However, whether this was added intentionally or simply the result of charging an ore containing lime is unknown, although a series of analysis by Morton(2,3) comparing Roman slags with Medieval slags, shows a definite trend for the Medieval slags to exhibit a higher Ca and lower Fe content. A consequence of adding lime is that the slag formed has a higher melting point so it was not until higher temperatures could be reached in medieval furnaces that it was possible to successfully smelt a higher lime containing charge and consequently improve the efficiency of the bloomery.
Ultimately, with the advent of the blast furnace in the 13th Century in parts of Europe, and in 1490s on the Weald, it was possible to reach such high temperatures that most of the iron in the slag could be displaced by calcium, and the iron itself became molten and could be tapped periodically from the furnace either to be cast into useful shapes, or into a 'pig' for refining. Refining of pig iron is necessary since the carbon content is around 4%, ten times or more than that of some bloomery iron. Sometimes, calcium was added to the furnace in the form of limestone, or sometimes it was already present in sufficient quantities in the ore, and also the charcoal ash provided CaO.
Table 4 compares bloomery slags with a typical blast furnace slag. All sites are in the Weald.
Table 4 Compositions of a bloomery slag and a blast furnace slag:
| Compound |
Sheffield
Furnace
Bloomery |
Sheffield
Forest
Bloomery |
Ashburnham
Blast
Furnace |
| FeO |
63.53 |
51.96 |
1.73 |
| Fe2O3 |
24.07 |
10.02 |
0.32 |
| FeS2 |
none |
none |
none |
| SiO2 |
5.64 |
25.15 |
49.63 |
| Al2O3 |
1.32 |
8.66 |
17.54 |
| CaO |
1.15 |
0.61 |
18.92 |
| MnO |
2.46 |
1.86 |
4.91 |
| MgO |
0.47 |
0.17 |
5.12 |
| P2O5 |
0.65 |
0.62 |
0.16 |
| TiO2 |
0.24 |
0.31 |
0.25 |
| H2O (combined) |
None |
None |
none |
| CO2 (combined) |
None |
None |
0.51 |
| SO3 (combined) |
0.20 |
0.17 |
0.32 |
Note, the blast furnace slag contains only about 2% total iron oxide whereas the bloomery slags in this example contain as much as 87% in total. Indeed, the slag is far richer in iron than the ore used to produce it. (This is possible as a greater quantity of ore produces a smaller quantity of slag). In some regions, following the introduction of the blast furnace, ancient bloomery slags were dug up and used as part of the charge to the blast furnace as they were so rich in iron. There is no record that this happened on the Weald, and much of the bloomery slag was used in road building from Roman times until the 19th Century, or simply left where it was.
Another point of interest in the comparison of bloomery and blast furnace slags is the much higher silica (SiO2) content of the blast furnace slag. The ability to treat ores containing high SiO2 was a further advantage of using the blast furnace. When we attempted to smelt a high silica ore (25-39% SiO2) from another region of the Weald, (Snape Wood, near Wadhurst), we could not produce a bloom, even although the ore contained 51% iron oxide. The slag produced was very viscous and did not flow. It contained 69% SiO2 and most of the iron had combined with this silica rather than producing free iron. Nevertheless, in the 19th Century, this ore was shipped to Shropshire for use in a blast furnace, although the high silica content would have resulted in a lot of slag being produced which increases fuel consumption.
A comparison of the composition of the slag produced from this high silica ore smelt, with slags produced using our usual lower silica Sharpthorne ore is given in Table 5.
Table 5 Comparison of compositions of WIRG Experimental slags:
| |
Sharpthorne
Smelt 15 |
Sharpthorne
Smelt 16 |
Snape High Si slag
Smelt 18 |
| Compound |
% (semiquant) |
% (semiquant) |
% (semiquant) |
| FeO |
42.4 |
39.0 |
13.7 |
| SiO2 |
30.4 |
28.4 |
68.7
|
| Al2O3 |
7.9 |
8.2 |
6.7 |
| CaO |
10.4 |
11.5 |
3.1 |
| K2O |
1.3 |
1.5 |
3.5 |
| Na2O |
0.9 |
0.8 |
0.9 |
| MnO |
2.1 |
2.2 |
0.7 |
| TiO2 |
0.5 |
0.3 |
1.2 |
| MgO |
2.5 |
2.7 |
0.8 |
| P2O5 |
0.9 |
0.8 |
0.46 |
| Cr2O3 |
Not detected |
Not detected |
Not detected |

© Wealden Iron Research Group 2003
| 
